Welcome to WordPress. This is your first post. Edit or delete it, then start writing!

Solutions for Brown Spots
WATCH NOW
Welcome to WordPress. This is your first post. Edit or delete it, then start writing!


DOWNLOAD HERE 1. Mohs Micrographic Surgery Success with Dr. Ogrich: Experience excellence in dermatological care with Dr. Lauren Ogrich, our board-certified dermatologist
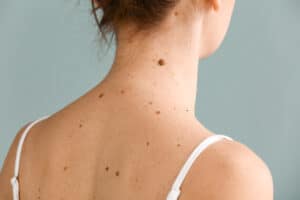
If you believe the researchers from London’s King’s College, people with lots of moles live longer. But not all moles are benign.
Be the first to receive news, promotions, and much more when you submit your email address.